A patient-led Good Controlled Trials Guide
Iain Chalmers UK RCT Registration Project
Contribution to a conference organized by the Socialist Health Association Involving patients and the public in the NHS’ London, 29 November 2002
How can the interests of users of health services be promoted most effectively?
Extracts from an unpublished paper written for a conference in Wales in 1976 “….Here again we come up against the difficulties of representing NHS users by proxy. A majority of NHS users are old people, many are mentally ill or mentally handicapped. Should organisations such as MIND and Age Concern therefore be particularly strongly represented on CHCs? “Or would direct elections provide more acceptable representation in spite of the fact that polling is likely to be low and that members elected on a political ticket might be inhibited when criticisms of an AHA was called for? Because CHCs currently offer the best hope for adequately representing the NHS user interests at local level, there is an urgent need for the Labour Movement to meet these uncertainties with practical suggestions.”
“A more satisfactory alternative would be the direct election of Authorities with responsibility for the provision of health and social services. However, present circumstances suggest that it may be some years before such Authorities could be established. Pending such a development, and working for its achievement, the Labour Movement could use its influence to press for more powerful Community Health Councils.”
Chalmers I. Democracy in the NHS. Paper prepared for the joint Wales TUC – Wales SMA Conference, Llandrindod Wells, 30 Oct l976.
An enduring question: How can the interests of users of health services be promoted most effectively? There are many answers to this question! But…it is a basic requirement that people should have ready access to the results of reliable research assessing the effects of health care interventions.
“Although the most important feature of CHCs is their local base, the creation of a national body would offer an opportunity to present matters of global concern… (It) should have the potential for conducting or commissioning its own research… it could collate evidence to indicate which drugs and therapies are beneficial, which useless and which positively harmful.” So have we made any progress towards this objective over the past quarter century? Did the Association of CHCs in England and Wales lobby for this? Does the National Institute of Clinical Excellence now fulfil this role?What has the NHS Research and Development Programme done?
NHS Centre for Reviews and Dissemination
NHS Health Technology Assessment Programme
National Electronic Library for Health
Clinical Evidence
So what do I want, as a patient? I want decisions about my care to be informed by systematic reviews of completed RCTs When there are uncertainties about the relative merits of my treatment alternatives I want to be invited to participate in any ongoing RCTs for which I may be eligible
Chalmers I. What do I want from health research and researchers when I am a patient? BMJ 1995;310:1315-1318.
My ‘RCT card’ – “Invite me to participate in all randomized controlled trials for which I am potentially eligible.” Medical Emergency Card (since 1989).
To whom should I look to promote and protect my interests? Research funders? Researchers? Research ethicists? Research funders & researchers Perverse incentives for researchers and institutions has led to poorly conducted research, addressing questions of no relevance to patients, and sometimes to scientific misconduct. Which perverse incentives?
- MONEY
- The scandal of poor medical research
- Academic competition and expectations
- ‘Requirements’ of health professionals, in training and in practice
- Research ethics “A particularly alarming offshoot of political correctness, with bogus academic credentials” Robert McIndoe, 2002 Savulescu J, Chalmers I, Blunt J. Are research ethics committees behaving unethically? BMJ 1996;313:1390-3.
Principles of good practice
All new research studies should be designed in the light of systematic reviews of relevant existing research evidence. All clinical trials should be registered at inception. The results of all clinical trials should be made public
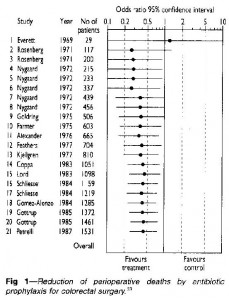
Systematic reviews are needed to identify useful treatments efficiently. Would any of you have agreed to participate in a placebo controlled trial of prophylactic antibiotics for colorectal surgery after 1975?
Systematic reviews are needed to identify harmful treatments efficiently
Was it necessary to recruit over 23,000 people with myocardial infarction to over 50 controlled trials of class 1 anti-arrhythmic drugs (all approved by research ethics committees) to discover that these drugs were lethal? Systematic reviews published at intervals during the 1980s showed that class 1 anti-arrhythmic drugs increased the risk of sudden death, yet additional trials were approved into the 1990s. At the peak of their use in the late 1980s, it has been estimated that class-1 anti-arrhythmic drugs given to people with heart attacks were causing between 20,000 and 70,000 premature deaths every year in the United States alone (Moore 1995). This yearly total of deaths is of the same order of magnitude as the total number of Americans who died in the Vietnam war. And some highly relevant studies were not published….. Trial of a class 1 anti-arrhythmic drug, reported in 1993, 13 years after it was completed.“Nine patients died in the lorcainide group and one in the placebo group…..When we carried out our study in 1980 we thought that the increased death rate that occurred in the lorcainide group was an effect of chance….. The development of lorcainide was abandoned for commercial reasons, and this study was therefore never published; it is now a good example of ‘publication bias’. The results described here ….might have provided an early warning of trouble ahead.”
Scientific and ethical misconduct:
- Not reviewing systematically what is known already before embarking on new research
- Not registering clinical trials at inception
- Not reporting the results of randomized trials
Research ethicists have failed to confront these problems, and have thus contributed to the avoidable suffering and deaths of millions of people using the health services. Chalmers I. Lessons for research ethics committees. Lancet 2002;359:174. Instead, research ethicists have:
- assumed that they own the moral high ground
- promoted micro-management of informed consent to treatment
- encouraged a view – unsupported by evidence – that treatment within clinical trials is more risky than treatment outside clinical trials
- promoted double standards on informed consent to treatment
“I need permission to give a drug to half of my patients, but not to give it to them all.” Richard Smithells 1975
“The clinician who is convinced that a certain treatment works will almost never find an ethicist in his path, whereas his colleague who wonders and doubts and wants to learn will stumble over piles of them.” Lancet Editorial 1990
If research funders, researchers and medical research ethicists can’t be relied upon to protect the best interests of people using the health services, who can be relied upon?!! The relevance of research evidence about the effects of health care seems unlikely to improve without greater lay understanding of, and involvement in, all stages of the research process. – Consumers in NHS Research Hanley B, Truesdale A, King A, Elbourne D, Chalmers I. Consumer involvement in the design, conduct and interpretation of randomised controlled trials. BMJ 2001;322:519-523.
Would a patient-led ‘Good Controlled Trials Guide’ help? Chalmers, Lancet 2000;356:774.
Commentaries on trials registered at www.controlled-trials.com could cover:
- the importance of the questions being addressed whether these had already been answered satisfactorily by previous research whether the design of the study was scientifically and ethically robust
- whether the primary outcomes chosen mattered to patients
- whether arrangements were in place for communicating the results of the research to those who had participated in it.
A scoping study conducted by the Health Service Research Unit, University of Aberdeen, on behalf of the Consumers in NHS Research Support Unit found: “widespread support for the concept of publicly available information on randomized controlled trials”. So what do you think? (And how will you help to ensure that people have ready access to the results of reliable research assessing the effects of health care interventions?)
